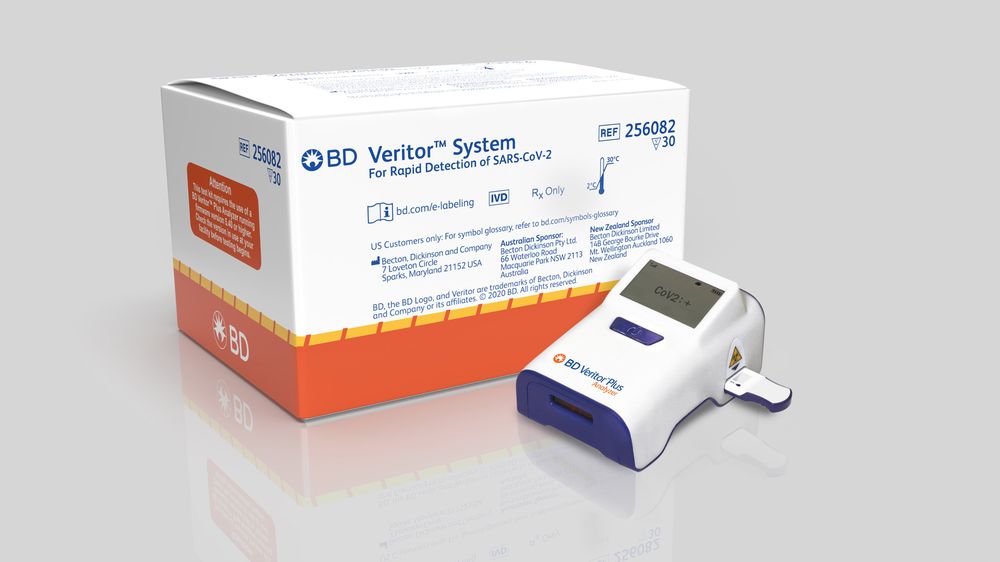
A COVID-19 test developed by Becton Dickinson and Co. has recently been cleared for use in countries that accept Europe’s CE marking which ensures that the product conforms to Europe’s safety and health standards.
This test works by quickly identifying proteins on the surface of the COVID-19 virus. These proteins, known as antigens, usually trigger an immune response in our bodies but no known antibodies currently exist that can fight this virus. This test will be run on the company’s small BD Veritor Plus System and be primarily sold to testing facilities, emergency departments, and clinics. It is expected that the test will be available for use in the European market by the end of October.
With COVID-19 on the rise again in Europe and the USA, the head of BD’s diagnostics for Europe says that this is really a game-changing introduction in Europe. The need for fast and accurate testing facilities is high. The company says that the test is 93.5% sensitive, how accurately it identifies infections, and 99.3% specific, meaning how accurately it identifies negative cases.
The company also said that the test is already available in the US and it will produce about 8 million tests globally each month by October. In the US, the BD Veritor Plus System costs around $250 to $300 with the tests being $20 each.



 8.5GB worth of K-Electric data leaked online by ransomware attackers
8.5GB worth of K-Electric data leaked online by ransomware attackers